The tetrazole analogue of the auxin indole-3-acetic acid binds preferentially to TIR1 and not AFB5
M. Quareshy, J. Prusinska, M. Kieffer, K. Fukui, A. J. Pardal, S. Lehmann, P. Schäfer, C. I. del Genio, S. Kepinski, K. Hayashi, A. Marsh and R. M. Napier
ACS Chemical Biology 13, 2585 (2018)
M. Quareshy, J. Prusinska, M. Kieffer, K. Fukui, A. J. Pardal, S. Lehmann, P. Schäfer, C. I. del Genio, S. Kepinski, K. Hayashi, A. Marsh and R. M. Napier
ACS Chemical Biology 13, 2585 (2018)
Abstract
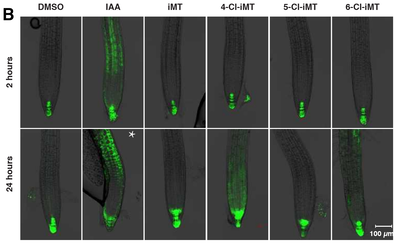
Auxin is considered one of the cardinal hormones in plant growth and development. It regulates a wide range of processes throughout the plant. Synthetic auxins exploit the auxin-signalling pathway and are valuable as herbicidal agrochemicals. Currently, despite a diversity of chemical scaffolds all synthetic auxins have a carboxylic acid as the active core group. By applying bio-isosteric replacement we discovered that indole-3-tetrazole was active by surface plasmon resonance (SPR) spectrometry, showing that the tetrazole could initiate assembly of the TIR1 auxin co-receptor complex. We then tested the tetrazole's efficacy in a range of whole plant physiological assays and in protoplast reporter assays which all confirmed auxin activity, albeit rather weak. We then tested indole-3-tetrazole against the AFB5 homologue of TIR1, finding that binding was selective against TIR1, absent with AFB5. The kinetics of binding to TIR1 are contrasted to those for the herbicide picloram, which shows the opposite receptor preference as it binds to AFB5 with far greater affinity than to TIR1. The basis of the preference of indole-3-tetrazole for TIR1 was revealed to be a single residue substitution using molecular docking, and assays using tir1 and afb5 mutant lines confirmed selectivity in vivo. Given the potential that a TIR1-selective auxin might have for unmasking receptor-specific actions, we followed a rational design, lead optimisation campaign and a set of chlorinated indole-3-tetrazoles was synthesised. Improved affinity for TIR1 and the preference for binding to TIR1 was maintained for 4-and 6-chloroindole-3-tetrazoles, coupled with improved efficacy in vivo. This work expands the range of auxin chemistry for the design of receptor-selective synthetic auxins.
Download
Link to the journal
Direct link to the preprint
Link to the arXiv