Tomographic docking suggests the mechanism of auxin receptor TIR1 selectivity
V. Uzunova, M. Quareshy, C. I. del Genio and R. M. Napier
Open Biology 6, 160139 (2016)
V. Uzunova, M. Quareshy, C. I. del Genio and R. M. Napier
Open Biology 6, 160139 (2016)
Abstract
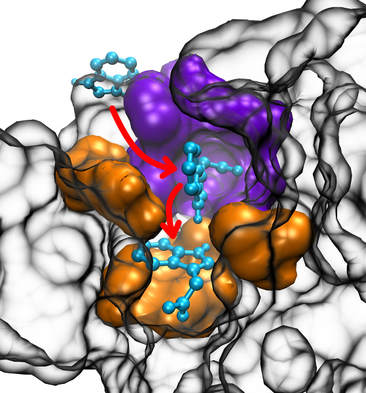
We study the binding of plant hormone IAA on its receptor TIR1 introducing a novel computational method that we call tomographic docking and that accounts for interactions occurring along the depth of the binding pocket. Our results suggest that selectivity is related to constraints that potential ligands encounter on their way from the surface of the protein to their final position at the pocket bottom. Tomographic docking helps develop specific hypotheses about ligand binding, distinguishing binders from non-binders, and suggests that binding is a three-step mechanism, consisting of engagement with a niche in the back wall of the pocket, interaction with a molecular filter which allows or precludes further descent of ligands, and binding on the pocket base. Only molecules that are able to descend the pocket and bind at its base allow the co-receptor IAA7 to bind on the complex, thus behaving as active auxins. Analyzing the interactions at different depths, our new method helps in identifying critical residues that constitute preferred future study targets and in the quest for safe and effective herbicides. Also, it has the potential to extend the utility of docking from ligand searches to the study of processes contributing to selectivity.
Download
Link to the journal
Direct link to the preprint
Direct link to the supplementary material
Link to the arXiv
Link to the bioRxiv
Link to the TomoDock code